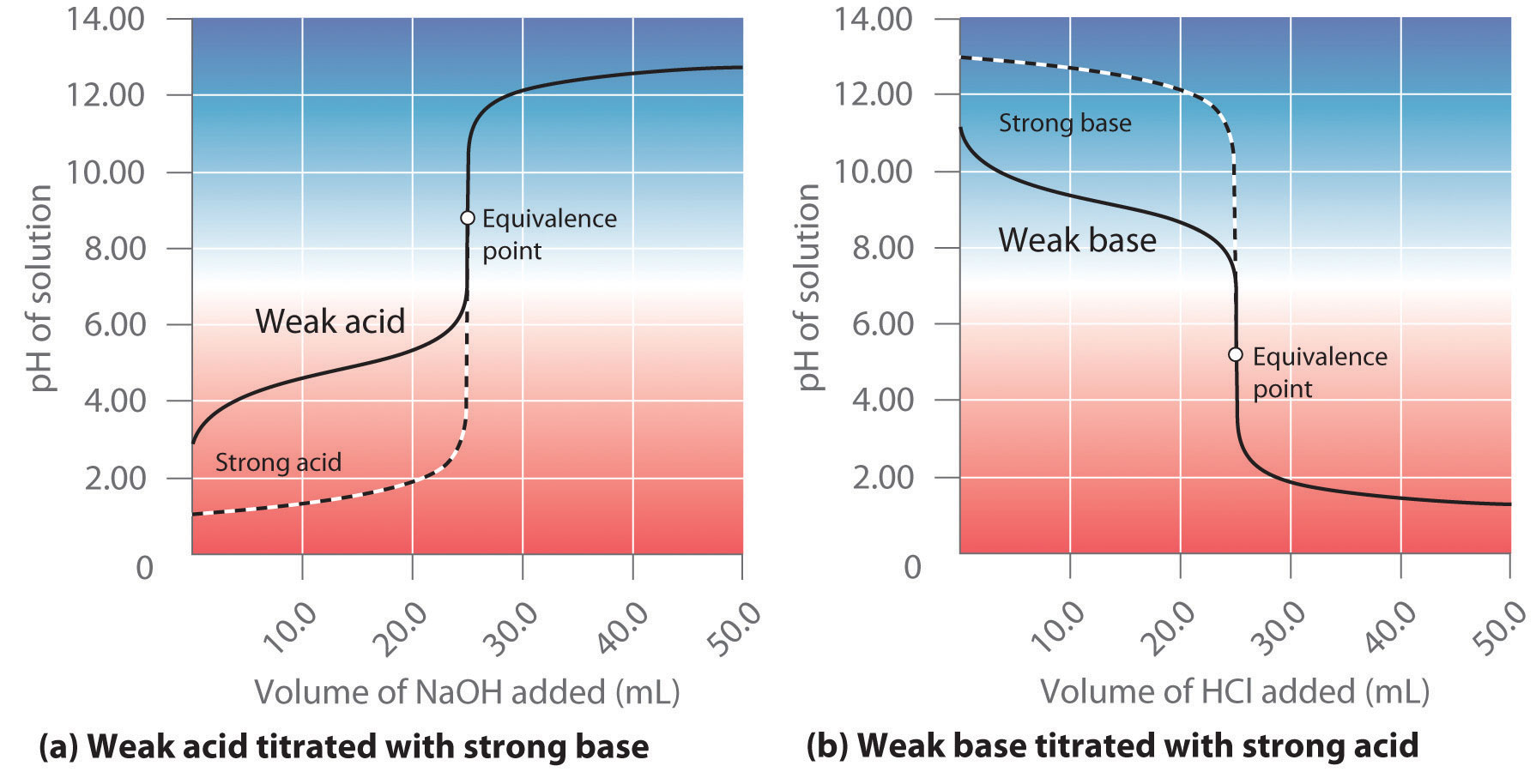
How Does Concentration Affect Titration .titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied.
from chem.libretexts.org
by far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount.the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and.titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.
15.6 AcidBase Titration Curves Chemistry LibreTexts
How Does Concentration Affect Titration by far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. the concentration of an acid solution can be determined by. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce How Does Concentration Affect Titrationthe objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and.by far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. titrations are done often to find out the concentration of one substance by reacting it. How Does Concentration Affect Titration.
From mavink.com
Dopamine Titration How Does Concentration Affect Titrationby far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. the concentration of an acid solution can be determined by.titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titrations are done. How Does Concentration Affect Titration.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint How Does Concentration Affect Titrationby far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. the concentration of an acid solution can be determined by.titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. in a titration,. How Does Concentration Affect Titration.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d How Does Concentration Affect Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied.titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.by far the most common use of titrations is in determining unknowns, that is,. How Does Concentration Affect Titration.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS How Does Concentration Affect Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied.the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and.titration is the slow addition of one solution of a known concentration (called a titrant). How Does Concentration Affect Titration.
From university.pressbooks.pub
15.7 AcidBase Titrations Chemistry Fundamentals How Does Concentration Affect Titration titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration.the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and.by far the most common use of titrations is in determining unknowns, that is,. How Does Concentration Affect Titration.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps How Does Concentration Affect Titrationthe objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. the concentration of an acid solution can be determined by.titration is the. How Does Concentration Affect Titration.
From webmis.highland.cc.il.us
AcidBase Titrations How Does Concentration Affect Titrationby far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount.the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. in a titration, a solution of known concentration (the titrant) is added to a solution. How Does Concentration Affect Titration.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts How Does Concentration Affect Titrationby far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. the concentration of an acid solution can be determined by. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. in a titration, a solution. How Does Concentration Affect Titration.
From chemwiki.ucdavis.edu
9B AcidBase Titrations Chemwiki How Does Concentration Affect Titrationby far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. the concentration of an acid solution can be determined by.titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. in a titration,. How Does Concentration Affect Titration.
From dixonspeould.blogspot.com
How To Find Pka On Titration Curve Dixon Speould How Does Concentration Affect Titrationtitration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. the concentration of an acid solution can be determined by.the objective. How Does Concentration Affect Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How Does Concentration Affect Titrationtitration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.by far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount.the objective of the titration is to find the volume of the base (of. How Does Concentration Affect Titration.
From www.youtube.com
Buffers and Titration Curves YouTube How Does Concentration Affect Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration.by far the most common use of titrations is in determining unknowns, that is, in determining. How Does Concentration Affect Titration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How Does Concentration Affect Titrationtitration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. titrations are done often to find out the concentration of one substance. How Does Concentration Affect Titration.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry How Does Concentration Affect Titrationby far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration.the objective of the titration is to find the volume of the base (of known concentration). How Does Concentration Affect Titration.
From bitesizebio.com
How Strong is Your Binding? A Quick Introduction to Isothermal How Does Concentration Affect Titration the concentration of an acid solution can be determined by. titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied.by far the most common. How Does Concentration Affect Titration.
From mirjamglessmer.com
Measuring the concentration of dissolved oxygen in sea water Part 3 How Does Concentration Affect Titration titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration.by far the most common use of titrations is in determining unknowns, that is, in determining the concentration or amount.titration is the slow addition of one solution of a known concentration (called a titrant) to. How Does Concentration Affect Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID6209773 How Does Concentration Affect Titrationtitration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. titrations are done often to find out the concentration of one substance. How Does Concentration Affect Titration.